Batteries for dummies part 1
For those of you who liked the blog posts „Arduino for dummies“ and “Logic for beginners” will also be interested in this one. As usual, I will explain everything in easy words as I am a complete beginner myself. Lets start with a simple definition of the battery:
A battery (or accumulator) is a storage for electrical energy that is rechargeable and that can submit the stored energy. A battery consists of several secondary battery cells. (Cells that cannot be recharged or only to a limited amount are called primary cells.)
You will have a battery by interconnecting secondary cells either in a series connection (this will increase electrical power) or in a parallel circuit (this will increase the capacity). Both circuits will increase the total energy content – which is defined as being the product of voltage and capacity. In conclusion: By interconnecting several secondary cells you will receive a battery with more total energy content.
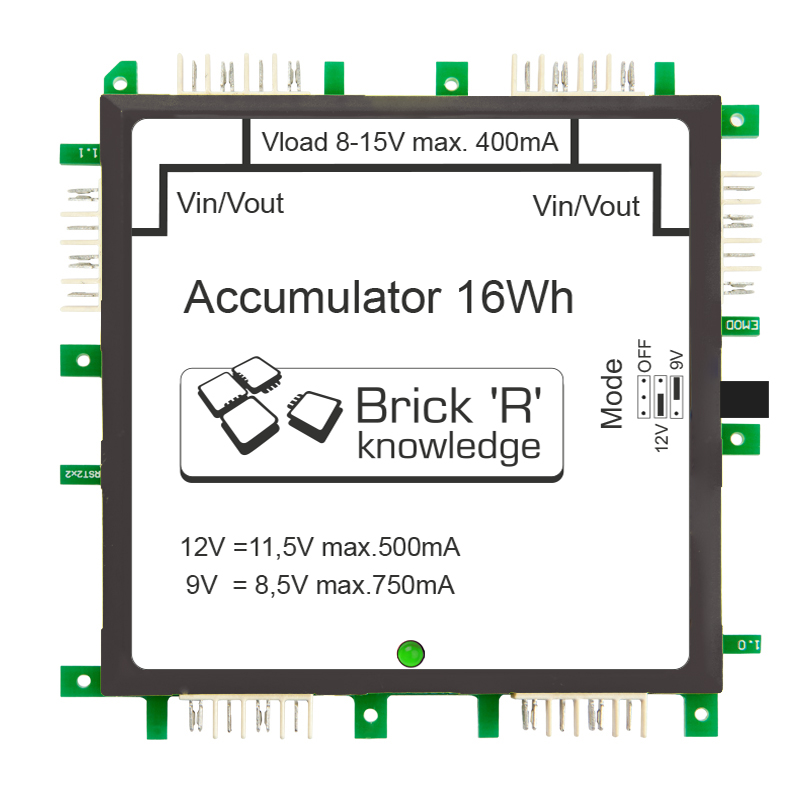
In order to understand everything more clearly we will have a closer look at the battery brick ALL-BRICK-0282.
The total energy content is indicated as watt-hours, the battery brick has 16 watt-hours. Here is a sample calculation: There are two smaller batteries in our brick battery. Each of it has a voltage of 3.8V and a capacity of 2300mAh (miliampere hours), that is 2.3Ah. This means, the battery can submit 3.8V including a current of 2.3A for one hour until it is empty.
Current x capacity = energy content
3.8V×2.3A =8.7W
As there are 2 batteries interconnected you need to double the result which will eventually lead to 17.4 watt-hours. The battery brick’s sticker has 16 watt-hours as a parameter. This difference – between reality and our calculation – exists because there are several other components inside the brick, for example, the microcontroller that also consumes power.
Enough of math today! This one is easier: Different kinds of batteries.
Although there are different kinds of batteries, the battery’s basic structure does not differ much from each other.
A battery includes two electrodes that are located in a special electrolyte. The electrolyte is either a liquid solution, a gel or a solid. You use different chemical substances for electrodes and the electrolyte. There are lead batteries, nickel cadmium batteries, nickel metal hydride batteries and lithium ion batteries. The battery brick is one of the latter where the positive electrode consists of lithium metal oxide and the negative electrode consists of graphite.
While discharging the positive electrode receives lithium ions that the negative electrode emitted. The positive electrode consists of a very light material to be able to receive the lithium ions.
A lithium ion battery has a very low self-discharge, namely, below 2% per months. The battery needs to be stored in an adequate way: when it is too hot, the battery will not last sufficiently. Lithium ions are used for laptops and e-cars.
This was it for today – the next part will include chemistry….stay tuned!

 en
en de
de es
es
